Impact of gut microbiota on colorectal anastomotic healing (Review)
- Authors:
- Published online on: April 14, 2025 https://doi.org/10.3892/mco.2025.2847
- Article Number: 52
-
Copyright: © Chen et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Introduction
Intestinal anastomosis is a fundamental surgical procedure performed in both emergency and elective settings to restore intestinal continuity following bowel resection. It is indicated in various conditions, including colorectal, bowel obstruction, blunt/penetrating abdominal trauma and intestinal perforation leading to peritonitis. Despite advancements in surgical techniques, including minimizing tissue damage, ensuring adequate vascularization, reducing anastomotic tension and employing optimal suturing methods, the rate of anastomotic leak (AL) has remained high, reaching up to 20% (1). AL is associated with increased morbidity, prolonged hospitalization and substantial healthcare costs, underscoring the urgent need for innovative therapeutic strategies to enhance anastomotic healing and reduce complications.
Previous studies have reignited interest in the role of the gut microbiota in intestinal wound healing and AL pathogenesis (2,3). The perioperative period is characterized by profound shifts in gut microbiota composition and function, driven by factors such as chemotherapy, radiotherapy, fasting, bowel preparation, antibiotic prophylaxis (4,5), surgical trauma, environmental exposure and ischemia-reperfusion injury (6). These disruptions can cause microbial dysbiosis, impaired gut barrier function and bacterial translocation, all of which have been implicated in AL pathogenesis (7). Despite increasing evidence linking the microbiome to tissue repair and immune regulation, the precise mechanisms through which microbial alterations influence anastomotic healing remain poorly understood. This knowledge gap highlights the urgent need for research into microbiome-based therapeutic approaches to significantly mitigate AL risks.
The present review hypothesized that the gut microbiome is a pivotal player in anastomotic healing and that targeted modulation of microbial communities during the perioperative period could reduce AL incidence. Understanding the interactions between gut microbiota, host epithelial cells and immune responses may enable the identification of predictive biomarkers and novel therapeutic targets. Potential translational applications include microbiome-based diagnostics for AL risk assessment, probiotics and prebiotics to restore microbial balance and targeted antimicrobial interventions to counteract pathogenic shifts. Such interventions directly improve patient outcomes by augmenting anastomotic healing, reducing postoperative complications and shortening recovery times. The present review explored the dynamic perioperative changes in gut microbiota, their impact on AL and emerging microbiome-targeted strategies to improve surgical outcomes.
2. Co-evolution of microbiota and host
The human gut harbors >100 trillion microbial cells, ~10-fold more than the combined number of human somatic and germ cells (8). Advances in metagenomic sequencing have facilitated the characterization of the gut microbiome, comprising complex microbial communities and their collective genomes. These approaches integrate high-throughput sequencing of specific 16S ribosomal RNA hypervariable regions and whole-genome shotgun sequencing to analyze microbial diversity and function (9,10). Most gut microorganisms reside in the lumen of the gastrointestinal tract, where they serve essential roles in metabolism, immune modulation and epithelial integrity. During infancy, the gut microbiome undergoes dynamic changes before stabilizing into four dominant bacterial phyla: Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria. The composition and prevalence of these microbes vary significantly based on environmental conditions, genetics, immune status, dietary habits and early encounters with infections or antibiotics (11). The gut microbial community consists of bacteria, viruses, fungi, archaea and protozoa, forming an intricate ecological network with profound physiological implications. The symbiotic relationship between gut microbiota and the host has co-evolved to support metabolic homeostasis, immune defense and intestinal barrier integrity. The microbiome serves a critical role in maintaining the host's nutritional and energy balance while also being essential for developing and sustaining a robust immune system (12). It facilitates nutrient assimilation by breaking down carbohydrates, fatty acids and proteins into bioavailable forms, thereby influencing nutrient metabolism and delivery to the host. This process includes regulating the activity of host genes related to nutrient transport and processing. Conversely, malnutrition can negatively affect both the innate and adaptive immune systems, as well as the microbiome itself (13). The gut microbiome also serves as a protective barrier against intestinal pathogens by producing antimicrobial compounds such as short-chain fatty acids (SCFAs), secondary bile acids and bacteriocins, which help maintain intestinal integrity (14,15). At the cellular level, SCFAs can directly or indirectly affect processes such as cell proliferation, differentiation and gene expression. Additionally, they act as ligands for G protein-coupled receptors (GPCRs), including GPR109A, GPR43 and GPR41, triggering anti-inflammatory signaling cascades (16). Among these, butyrate enhances intestinal barrier function in IPEC-J2 cells by selectively upregulating tight junction proteins and activating the Akt signaling pathway (17). Despite these insights, the precise mechanisms by which the gut microbiome confers resistance to pathogen colonization remain incompletely understood. These mechanisms likely include the production of antimicrobial substances, competition for nutrients, maintenance of intestinal barrier integrity and bacteriophage-mediated bacterial regulation. The interplay between the host's genetic makeup and microbiota composition significantly influences susceptibility to diseases (18). Disruptions in microbial populations, including the depletion of certain beneficial bacteria or significantly reduced microbial diversity, can elevate the risk of infections and complications, such as AL.
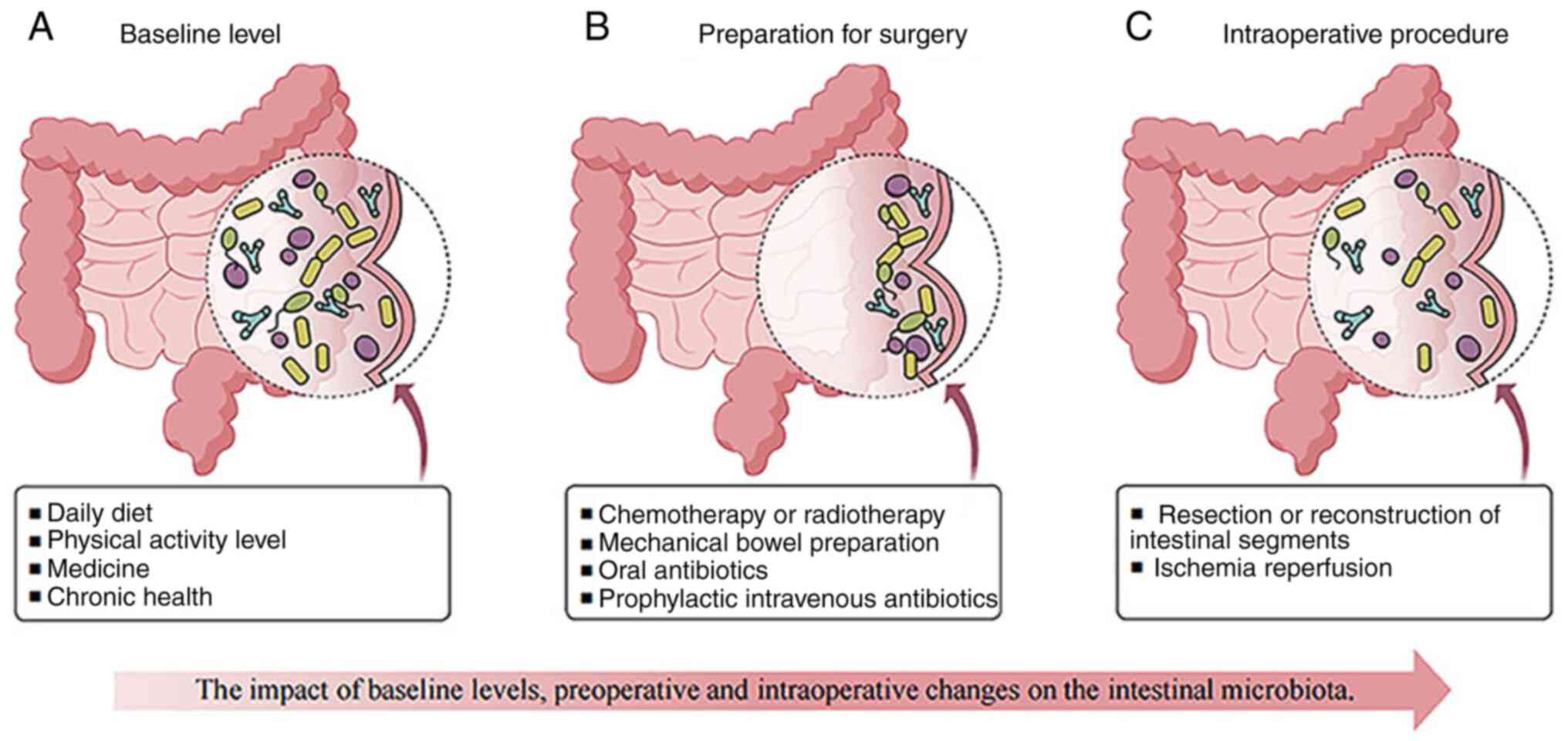
3. Perioperative gut microbiota changes associated with AL
Baseline alterations in gut microbiota related to AL
As aforementioned, the human gut harbors numerous microorganisms. The gut microbiota-host interaction creates a balanced microecological environment crucial for maintaining normal biological functions. The gut microbiota composition can be altered through surgical interventions but also by changes in host physiology and immune responses (19). Several factors can influence gut microbiota composition and function, such as dietary habits, physical activity levels, medications and chronic health conditions (20,21). Diet also serves a fundamental role in determining the structure and function of gut microbial communities (22). Additionally, specific dietary components can directly affect the gut microbiota or indirectly modulate it by influencing the host's metabolism and immune system. Regulatory T cells, also known as Treg cells, are essential to sustain intestinal balance. SCFAs, essential for Treg cell homeostasis, are produced through bacterial fermentation of dietary fibers (23,24). A deficiency in Treg cells leads to inflammation, illness (including type 1 diabetes mellitus, multiple sclerosis, rheumatoid arthritis and inflammatory bowel disease) and an imbalance in the gut microbiota composition. Dietary components can interfere with the protective role of the intestinal barrier. Western-style, high-fat, and low-fiber diets impair intestinal barrier function in animal models. However, fiber supplementation may help restore barrier integrity (25-27). In addition to diet, physical activity significantly influences the gut microbiota composition (28), but its effects depend on intensity. While moderate exercise can be beneficial, intense physical activity may compromise epithelial integrity, leading to increased intestinal permeability, bacterial translocation and inflammation (29,30). Moreover, certain medications including opioids and nonsteroidal anti-inflammatory drugs (NSAIDs) can adversely affect anastomotic healing by altering the gut microbiota. A rat study demonstrated that morphine administration impaired surgical site healing and increased AL risk, which was associated with the presence of collagenase-producing Enterococcus faecalis in anastomotic tissue (31). Furthermore, alterations in the gut microbiota composition have been linked to different chronic diseases and lifespan variations in both human and animal studies. Conditions such as type 2 diabetes (32), depression and chronic stress (33) and liver injury (34) have all been associated with microbiota imbalances.
Preoperative gut microbiota changes associated with AL
Preoperative preparation can significantly alter the gut microbiome, especially around the surgical anastomosis site (35). While achieving a complete pathological response following neoadjuvant chemoradiotherapy is widely recognized as beneficial in cancer treatment, its impact on anastomotic healing or leakage rates remains unclear. Radiation therapy has been shown to alter the microbiome composition, but there is no conclusive evidence linking it to an increased AL risk (36,37). A previous study developed a rat AL model to investigate the effects of preoperative radiation on AL. The rats underwent distal colon resection after radiation exposure, mimicking treatment protocols for advanced rectal cancer. Subsequently, Pseudomonas aeruginosa, a common colonizer of the radiated intestine, was introduced into their intestines. The irradiated intestinal tissues exhibited a considerably higher AL risk when infected with P. aeruginosa (37). However, a previous systematic review and meta-analysis concluded that neoadjuvant chemotherapy is not significantly associated with an increased incidence of AL or other adverse postoperative outcomes (38).
Surgeons recognize that the physical trauma of surgery, such as incisions, tissue dissection, organ removal and vascular reconnection, heightens a patient's susceptibility to infections. Current standard clinical protocols aim to minimize microbial presence and prevent postoperative complications (39,40). Common preoperative strategies include mechanical bowel preparation (MBP) with laxatives, administration of oral and/or intravenous antibiotics and topical application of disinfectant solutions to sterilize the intestines and skin (41). Despite these measures, the gut microbiota remains a potential reservoir of pathogens that may contribute to post-surgical infections, particularly in intestinal surgery. The effects of preoperative bowel preparation on gut microbiota preservation and bacterial phenotype transformation are still not fully understood and warrant further investigation. Research indicates that MBP alone induces transient microbiome alterations, the magnitude of this effect is minimal, and the gut microbiome generally returns to its baseline state within 10 days following a colonoscopy. However, patients undergoing systemic bowel preparation (SBP), which includes a combination of MBP and oral antibiotics, experience more pronounced disruptions in microbiome composition, requiring at least 30 days for the gut microbiome composition to reach the initial state (4). MBP functions by removing solid fecal matter and reducing bacterial load in the colon, leading to temporary shifts in microbial diversity. Specifically, it decreases the abundance of beneficial bacteria such as Bifidobacterium and Lactobacillus, while increasing the abundance of Escherichia coli and Staphylococcus. When combined with SBP, microbial diversity is further reduced, nearly eliminating obligate anaerobes while favoring facultative anaerobes such as E. faecalis. Additionally, SBP has been associated with increased antibiotic resistance (40). Moreover, the indiscriminate use of preoperative intravenous antibiotics may not provide any substantial advantages in reducing postoperative infections (42). Emerging evidence suggests that almost 50% of severe post-surgical infections involve antibiotic-resistant microbes. E. faecalis and P. aeruginosa frequently colonize leaking anastomoses, despite the use of strong broad-spectrum antibiotics (43). In rats, localized antibiotic application, rather than systemic intravenous administration, effectively eliminates E. faecalis and lowers AL risk in colorectal surgery (44). A combination of preoperative oral antibiotics and MBP has been shown to reduce Al incidence of colorectal surgery by nearly 50%. However, neither MBP alone nor oral antibiotics alone provide significant protection against AL (Table I) (45,46). Furthermore, the impact of MBP on microbiome composition and postoperative infection risk, including AL, may be influenced by individual patient characteristics such as body mass index and metabolic profile. A randomized controlled trial investigating microbiome alterations following MBP in patients undergoing elective colorectal surgery found substantial reductions in overall bacterial counts, particularly in Clostridium, Bifidobacterium, Lactobacillus and Enterobacteriaceae, with no significant reductions noted in Enterococcus and Staphylococcus (47). Thus, the benefits of bowel preparation remain controversial.
Intraoperative gut microbiota changes associated with AL
Surgical resection of diseased, obstructed or ischemic bowel often requires complex procedures to restore intestinal continuity. These interventions significantly affect the gut microbiome, leading to substantial compositional shifts (53-55). Intestinal manipulation induces tissue damage and inflammation, which contribute to AL. Specifically, matrix metalloproteinase-9 is upregulated after surgery, facilitating leukocyte migration and inflammation, thereby impairing anastomotic healing. Additionally, intestinal ischemia-reperfusion (IIR) injury triggers dynamic changes in colonic microbiota. Although the precise mechanisms remain unclear, IIR induces inflammation and oxidative stress. The gut microbiota typically provides colonization resistance, preventing the overgrowth of indigenous pathogens, which suggests the suppression of potentially pathogenic commensals within the microbial community (56). However, IIR disrupts the intestinal mucosal barrier, reducing commensal bacteria and fostering pathogenic proliferation, ultimately exacerbating intestinal inflammation (57). In a mouse model, mesenteric ischemia-reperfusion resulted in increased E. coli levels in the ileum and colon while reducing the Lactobacillus levels, leading to gut barrier dysfunction and bacterial translocation (58). Fang et al (59) conducted a clinical study analyzing 332 fecal samples from 129 individuals (50 with ulcerative colitis and 79 with Crohn's disease). Their findings demonstrated that intestinal surgery reduces microbial diversity and metabolite concentrations in patients with inflammatory bowel disease, with long-lasting effects (including reduced diversity of microbes and metabolites, and further increase of the instability of the gut microbiome in patients with IBD). Previous research corroborates that preoperative interventions and surgical stress disrupt microbiota balance, causing metabolic disruptions and increasing susceptibility to AL (60). Therefore, surgical reconfiguration fundamentally alters the gut microbial ecosystem, impacting digestion, nutrient absorption, and immune function (55). The extent to which these alterations contribute to postoperative AL warrants further investigation (Fig. 1).
4. Interaction between gut microbiota changes and factors affecting intestinal wound healing
AL, a serious postoperative complication, is associated with both increased local recurrence rates and reduced disease-free survival in colorectal cancer patients compared with non-leakage cases (5). It has been a longstanding worry for surgeons, despite their meticulous surgical technique. AL remains unpredictable, occurring even in patients without identifiable risk factors. Efforts to identify high-risk patients for anastomotic failure after colorectal surgery have led to extensive research on potential contributors to anastomotic failure. A prospective, multicenter snapshot study by Gao et al (61) analyzed 1,854 patients undergoing right hemicolectomy for colorectal cancer. Multivariate analysis identified side-to-side anastomosis, intraoperative blood loss >50 ml, and neoadjuvant chemotherapy as independent risk factors for AL (61). Importantly, the gut microbiota significantly influences various factors affecting intestinal healing (including the microbial barrier, immune regulation, tissue repair and alterations in the metabolic microenvironment), making it a potential target for novel prevention and treatment strategies. Following colorectal anastomosis, tissue repair progresses through four stages: Hemostasis, inflammation, proliferation and wound remodeling (62). AL is influenced by a range of biological processes, including gut microbiota composition, inflammation, host genetics and immune responses. Among these, the gut microbiota serves a particularly crucial role in intestinal wound healing (63,64).
Inflammation
During the inflammatory phase, the gut microbiota can promote or hinder wound healing by modulating cellular activation and fibrosis (65,66). While the precise mechanisms remain incompletely understood, studies suggest that lipoxin A4 and annexin-1 contribute to IL-10-dependent attenuation of inflammation in germ-free mice (67). Notably, administering lipoxin A4 or annexin-1 peptides to conventional mice mitigates tissue damage, TNF release and mortality following IIR injury (67). Various anti-inflammatory treatments (for instance, prolonged use of synbiotics, prebiotics and probiotics may modulate inflammatory status and potentially impact perioperative outcomes) have been explored for their role in preventing AL in animal and human studies. However, the results have been highly variable. Suppressing inflammation alone does not appear to prevent AL, as anti-inflammatory medications have failed to reduce its incidence (68). Interestingly, NSAIDs may disrupt microbial function, leading to dysbiosis and impaired anastomotic healing. Postoperative NSAID use has been correlated with increased AL rates, possibly due to its effects on the gut microbiota (69). Although NSAIDs are primarily prescribed for pain management rather than inflammation reduction, NSAIDs have been consistently associated with higher AL incidence (70,71).
Host genetics
While some single nucleotide polymorphisms (SNPs) have been identified in association studies, evidence for the influence of host genetics on AL is limited, and this research field remains in its early stages. COX-2, also known as Ptgs2, serves a crucial role in intestinal wound healing following colorectal surgery, primarily through its effects on angiogenesis, which is essential for anastomotic healing (72,73). A specific SNP in human PTGS2 (-765G>C; rs20417) has been associated with reduced COX-2 expression levels and an increased risk of AL (74,75). Studies on mice deficient in COX-2 have demonstrated a higher incidence of AL and mortality, which can be partially mitigated by the administration of prostaglandin E2 (PGE2). As the primary product of COX-2, PGE2 is essential for preserving intestinal homeostasis (74).
Intestinal immune regulation
The intestinal barrier and mucosal immune system work together to maintain a delicate balance between the host and external microorganisms, regulating immune responses to beneficial organisms while protecting against harmful pathogens. Increasing evidence highlights the crucial role of the gut microbiome in immune system maturation. The gut microbiota influences intestinal wound healing and epithelial repair through various molecular pathways (76). For example, gut microbes affect cellular immunity by modulating lymphocyte polarization, transportation and cross-reactivity. The microbiota can influence the adaptive immune system in various ways, including the bystander effect on T cell populations and molecular mimicry impacting antigen responses (77,78). Intestinal symbionts contribute to the development of gut-associated lymphoid tissue, secretory IgA and Th17 cells. It has been previously reported that cytokine levels fluctuate in the blood or peritoneum of patients undergoing colorectal resection, suggesting that the interleukin (IL)-Th17 pathway is a contributing factor to AL. Transforming growth factor-beta and IL-6 stimulate the differentiation of naïve CD4+ T cells into Th17 cells by increasing the expression of IL-23 and IL-1 receptors, which are essential for Th17 cell expansion and survival (79). These factors help maintain intestinal homeostasis and the host-microbiome balance (76). The presence of resident microbiota, influenced by the gut microbiome, affects Th17 cell development (80) and may contribute to AL (81). Additionally, microbial signaling pathways serve a crucial role in wound healing. Pattern recognition receptors, such as toll-like receptors (TLRs), are key components of the innate immune system (82) and recognize pathogen-associated molecular patterns from microorganisms (83,84). TLRs, expressed in both immune and non-immune cells, detect gut microbial elements such as lipopolysaccharides (LPS) and flagellin (85), which help maintain intestinal homeostasis and promote epithelial healing. Rakoff-Nahoum et al (86) demonstrated that under normal homeostatic conditions, symbiotic bacteria are recognized by TLRs. Mice deficient in TLR2, TLR4 or MyD88 exhibit exacerbated acute gastrointestinal epithelial injury and inflammation. Cox-2 serves a key role in intestinal inflammation and healing. Its activity is regulated by TLR4 signaling and is essential for intestinal mucosal regeneration. Furthermore, TLR4-deficient mice fail to upregulate Cox-2 expression levels in response to epithelial damage (73). Cox-2 is expressed by both intestinal epithelial cells and lamina propria macrophages in a TLR4- and MyD88-dependent manner (73). Overall, these findings suggest that direct interactions between gut bacteria and the immune regulatory mechanisms of the intestinal lining may enhance wound healing and tissue repair. Intestinal wound healing should be considered in conjunction with the microbiome, host genetic composition, immune regulation and their relationship with the perioperative inflammatory state, as well as the influence of interdependent factors.
5. Depletion of factors promoting anastomotic healing
Reduced microbial diversity
The gut microbiota serves a crucial role in wound healing. In 1955, Cohn and Rives (87) first proposed that the intestinal microbial composition influences AL development. Disruptions in gut microbiota diversity are frequently observed due to preoperative or perioperative interventions, as well as surgical stress. Such disruptions can lead to metabolic disarray and a decreased ability to resist pathogenic invasion, potentially increasing the risk of AL (60). The significant depletion of beneficial bacteria in anastomotic tissue may facilitate the colonization of pathogenic microbes, ultimately hindering the recovery process (88,89).
Changes in gut microbiota metabolites
Imbalances in the gut microbiome also alter the production of metabolites such as SCFAs and bile acids, as well as bacterial components such as LPS and peptidoglycan. These modifications can cause disturbances in the intestinal barrier, impair hormonal regulation and trigger immune dysregulation, ultimately impacting gut function and overall health (90). Butyrate, a key SCFA, is the primary energy source for colonic epithelial cells and serves a crucial role in promoting cell proliferation and maintaining barrier integrity. It also has anti-inflammatory properties helping to reduce pro-inflammatory cytokines (91,92). Molecular studies suggest that butyrate lowers the AL risk by suppressing the proliferation of P. aeruginosa (93,94). Formyl peptide receptors (FPRs), along with reactive oxygen species (ROS) and reactive nitrogen species, are considered to contribute to the beneficial effects of gut bacteria on intestinal regeneration and healing. Butyrate-producing bacteria exhibit strong protective effects and may regulate extracellular signal-regulated kinase pathways via FPR-mediated signaling, which triggers ROS production in intestinal epithelial cells. These processes improve gut repair and regeneration, helping to maintain mucosal integrity and function (62,95,96). Additionally, research indicates that specific gut microbiota can confer infection resistance by converting host bile salts into metabolites that inhibit Clostridioides difficile (97). However, bacteria can trigger systemic inflammation and sepsis (98). In sepsis, LPS-induced inflammation is a crucial pathological occurrence that disrupts metabolic and immune functions (99-101). Furthermore, bacterial peptidoglycan has been shown to stimulate the production of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-10, contributing to tissue damage and immune dysregulation (102). In a rat model, injection of peptidoglycan-polysaccharide from Streptococcus pyogenes into the distal colon induced chronic granulomatous colitis (103). These studies provide new insights into how gut microbial derivatives and metabolism influence postoperative healing and AL risk.
Decline in angiogenesis regulation
Probiotics serve a crucial role in aiding wounds at various stages of intestinal recovery (104). In the gut, symbiotic bacteria regulate the formation of new blood vessels (104), which helps reduce intestinal inflammation and promotes mucosal tissue healing during the inflammatory phase of wound repair. This process occurs through the signaling of the vascular endothelial growth factor receptor (VEGFR). For example, the yeast Saccharomyces boulardii modulates angiogenesis by adjusting VEGFR signaling. This regulation decreases intestinal inflammation and supports mucosal tissue repair. Inflammation can have both beneficial and detrimental effects on intestinal AL and angiogenesis is a crucial component of the inflammatory response necessary for mucosal remodeling during recovery (105). In 2002, Stappenbeck et al (106) conducted a study on rodents highlighting the essential role of the microbiome in rebuilding the mesenchymal microvascular system by involving Paneth cells, key components of the innate immune response. Their research revealed that Bacteroides thetaiotaomicron can increase the capillary network in the small intestine two-fold by acting on Paneth cells, thereby promoting angiogenesis (106).
6. Intestinal barrier disruption
Perioperative procedures for colorectal surgery can lead to a reduction in bacterial populations and dysbiosis, which can further cause intestinal barrier dysfunction. The roles of the four intestinal wall layers (the mucosa, submucosa, muscularis propria and serosa) in anastomotic healing remains an area of ongoing research. During colorectal resection, all four layers are transected before the formation of an anastomosis. Nevertheless, the mucosa and submucosa are in close proximity to the intestinal microbiota, making them particularly relevant to healing.
Submucosa
The submucosa, composed of connective tissue, has the highest tensile strength among the four intestinal layers. The submucosa is recognized as the main structural component responsible for anastomotic healing (107). As the most resilient fibrous layer, it consists mainly of elastin fibers and collagen, making it the most resistant of these layers to mechanical stress (108). This layer serves as a major source of fibroblasts, which are activated after gastrointestinal surgery to produce and release collagen. Bacterial adhesion to collagen is a crucial factor in anastomotic healing (109,110). Mechanistic studies have discovered that imbalanced gut microbiota can inhibit epithelial cell migration and repair by interfering with integrin α2β1 or laminin-332 in the extracellular matrix, thereby suppressing Rac1 expression (111).
Mucus layer
Considering that bacteria reside within the colonic mucus; the mucosa serves a more significant role in healing than previously considered (15). The gastrointestinal tract relies on mucus as its first line of defense against external factors. Preoperative bowel preparation can alter the composition and production of the protective mucus layer, potentially triggering bacterial translocation. Everard et al (112) demonstrated that mucus-degrading bacteria, such as Akkermansia muciniphila, which inhabit the mucus layer, can restore gut barrier function. These bacteria influence the mucus layer by increasing mucus production and stimulating the secretion of specific antimicrobial proteins, which help reduce weight gain, fat accumulation and low-grade inflammation. Reduced mucus levels expose collagen at the anastomotic site, enabling pathogens to colonize and produce collagenases (113).
Mucosal layer
Li et al (114) demonstrated that the gut microbiota enhances the mucosal layer's ability to defend against infections. Indigenous microorganisms inhabit microbial niches, competing with intestinal pathogens and opportunistic bacteria to avert infections. The intestinal epithelium serves as a physical barrier, forming a monolayer of interconnected biofilms that prevent bacterial invasion and the translocation of pathogens and microbial products (115,116). Additionally, intestinal epithelial cells possess inducible innate defense mechanisms that enable rapid responses to pathogenic challenges. These include the secretion of salt, water, antimicrobial peptides and complex glycoproteins, such as mucins (117). Mucins, produced by mucosal epithelial cells, form a protective barrier that limits the penetration of environmental substances into the epithelial layer (15). Certain probiotic lactobacilli species adhering to intestinal epithelial cells can rapidly induce the expression of the eukaryotic MUC3 mucin gene. This secreted mucin inhibits the adhesion of intestinal pathogens to epithelial cells, enhancing barrier function (89). A foundational study reported that Muc2 mucin gene knockout mice exhibited increased leukocyte infiltration, reduced collagen deposition and impaired angiogenesis. Consequently, colonizing bacteria came into direct contact with intestinal epithelial cells, exacerbating inflammation and tissue damage (118). Bacteria adhere to mammalian cells via the AIDA-1 adhesin protein. A clinical study reported that patients with AL had a higher abundance of mucin-degrading bacteria from the Lachnospiraceae and Bacteroidaceae families at the anastomotic site. This bacterial composition may serve to predict AL (119). Tight junctions, which function as intracellular boundary structures, serve a crucial role in preserving the integrity of the intestinal epithelial barrier. They regulate the passage of solutes and molecules while preventing the translocation of harmful substances, such as lipids and microbial peptides, from the gut into the bloodstream (120). Previous findings indicate that the microbiome serves a crucial role in effective wound healing, influencing systemic physiological processes. Activation of β-catenin signaling through interactions between the microbiome and epithelial cells is essential for regulating epithelial cell proliferation (121). This highlights the complex molecular and cellular interactions required for repairing intestinal epithelial repair, involving host cells, luminal growth factors and the gut microbiota (122,123). During surgical anastomosis, the proximity of two suture lines may lead to an imprecise circumferential connection between the proximal and distal segments. Complete epithelial coverage across the anastomotic site is a critical prerequisite for early-stage healing. A. muciniphila serves a key role in this process by activating pathways that enhance epithelial cell movement and proliferation (124). Bacterial fermentation of dietary fibers produces SCFAs such as acetate, propionate, butyrate, valerate and isovalerate, which serve as the primary energy source for colonic epithelial cells. SCFAs also exert a direct nutritional impact on the colonic mucosa, promoting its integrity and function (16). In vitro research has demonstrated that A. muciniphila and Bacteroides fragilis enhance intestinal epithelial integrity despite being anaerobic organisms (125). Of note, these bacteria can survive in aerobic environments, a critical feature given that surgical procedures can temporarily elevate oxygen levels in the lower gastrointestinal tract, reducing the number of obligate anaerobes. Mouse studies have replicated these bacterial effects, demonstrating faster mucosal re-epithelialization and improved healing outcomes (124,126).
7. Common pathogens associated with AL
Under the stress of surgery or perioperative treatment, gut-colonizing symbiotic and pathogenic microbes can potentially develop aggressive and toxic tissue-degrading phenotypes (producing collagenases that directly degrade collagen fibers and activate host-derived proteases, collectively contributing to tissue breakdown). Previous research has explored the cause-and-effect relationship between AL and the intestinal microbiome, revealing that anastomotic damage results in notable alterations in the structure and operation of the microbiome associated with anastomotic tissues. Komen et al (127) identified E. faecalis and P. aeruginosa as the predominant pathogens at leakage sites, showing significant collagenolytic activity (127). Tissue-degrading bacteria, such as E. faecalis, P. aeruginosa, Pseudomonas putida and Fusobacterium nucleatum, produce collagenases that break down collagen and activate host proteases such as MMP9 (42,128).
E. faecalis
Collagens I and IV serve critical roles in maintaining and repairing the extracellular matrix. E. faecalis is of particular interest due to its collagen-degrading activity, which may serve as a potential distinguishing factor between AL and non-AL. Significant upregulation of Enterococcus and Enterobacter species has been observed in the intestinal mucosa near colonic anastomoses in rats (46). As a major cause of enterococcal bacteremia, E. faecalis is a common pathogen causing nosocomial bloodstream infections. It is known for its strong adhesion to extracellular matrix proteins such as fibronectin, laminin and type IV collagen (129,130). Additionally, E. faecalis has been shown to induce the activation and cleavage of intestinal MMP9, which is associated with AL, in a GelE/SprE-dependent manner (42). These findings suggest that E. faecalis may impair anastomotic healing through a dual mechanism.
P. aeruginosa
A study conducted by Schardey et al (131) was the first to suggest the potential involvement of P. aeruginosa in AL. One of its most critical virulence factors is the type III secretion system (T3SS). Jin et al (132) reported that MexT regulates T3SS through MexS and PtrC, and mutations in the mexT gene contribute to P. aeruginosa virulence transformation, serving a crucial role in cytotoxicity. Sequence analysis of P. aeruginosa at anastomotic sites has revealed an SNP mutation in the mexT gene, conferring swarming ability, enhanced collagenase activity and an epithelial-destructive phenotype (37). Consequently, bacteria residing at the anastomotic site can detect subtle changes in cytokines, chemokines and ischemic byproducts and respond by increasing their virulence, particularly through the expression of collagenolytic phenotypes (Fig. 2).
8. Preventing AL from a microbiome perspective
Previous studies highlight the essential role of the gut microbiota in maintaining intestinal health, protecting the intestinal barrier and supporting normal digestive functions (133,134). As the microbiome serves a crucial and pathogenic role in AL etiology and pathogenesis, it represents a promising target for intervention. Preventing AL may involve identifying high-risk patients with unfavorable microbiome compositions, including an overabundance of collagenase-producing pathogens. Preventative strategies include preoperative prehabilitation through dietary modifications, personalized antibiotic treatments (oral, intravenous or enema), microbiome restoration and/or adherence to Enhanced Recovery After Surgery (ERAS) protocols (41).
Healthy eating habits
Unlike the host genome, the microbiome is highly adaptable to environmental and dietary influences. Among these factors, dietary habits profoundly shape microbial composition. Preoperative dietary preparation with a low-fat, high-fiber diet has been shown to enhance anastomotic healing by modulating the microbiome. Studies have explored how short-term dietary interventions can counteract the negative effects of high-fat Western diets on anastomotic healing in mice. A Western diet significantly disrupted both the gut microbiota composition and anastomotic healing, whereas a preoperative low-fat, high-fiber diet restored microbial diversity and improved outcomes (135). Guo et al (136) further confirmed that a preoperative low-fat, high-fiber diet enhances microbial diversity postoperatively and accelerates anastomotic healing. This dietary adjustment can mitigate the harmful effects of a Western diet, leading to improved survival rates after surgery (137). Furthermore, modifying the gut microbiome using dietary inulin and 5-aminosalicylic acid can strengthen the intestinal barrier and prevent anastomotic tumors and metastatic spread in mice (138). Mice receiving diets supplemented with inulin, galacto-oligosaccharides (GOS) or cellulose for 2 weeks before colonic surgery exhibited improved results as inulin and GOS increased butyrate production and improved anastomotic healing. Therefore, dietary supplementation with inulin and GOS can strengthen intestinal barriers and promote recovery in mice (139).
Personalized bowel preparation
Perioperative procedures disrupt the gut microbiome, leading to reduced microbial diversity and an increased risk of opportunistic pathogen overgrowth (4,49). Localized decontamination strategies have been successfully employed to prevent colorectal AL. Applying a non-absorbable antibiotic mixture of polymyxin B, gentamicin and vancomycin locally every 6 h for 5 days postoperatively prevented AL (140). Schardey et al (141) also confirmed that local antibiotic decontamination is highly effective in preventing AL in rectal surgery. Scarborough et al (46) analyzed data from the American College of Surgeons National Surgical Quality Improvement Program, studying 4,999 patients. They found that a combination of preoperative oral antibiotics and MBP reduced the incidence of AL in colorectal surgery from 5.7 to 2.8%, compared with patients who received no preparation. Neither oral antibiotics nor MBP alone were effective in reducing AL rates (46). Further research indicates that a double-dose bowel cleansing regimen has a lesser impact on the intestinal microbiome than a single dose, leading to a faster microbial recovery period, making it a more suitable clinical application (43). The bowel preparation protocol offers two options: a split-dose regimen (1 L twice) or a single-dose regimen (2 L once). While the total volume remains identical, the administration frequency differs. Laboratory studies in rats have shown that preoperative treatment and surgery result in an increase in facultative anaerobes and a depletion of SCFAs (21,58). This highlights the importance of preoperative administration of SCFAs, such as butyrate, to counteract microbial imbalances. Additionally, surgical procedures, cancer treatment, antibiotics and painkillers can reduce Lactobacillus levels at anastomotic sites. This decrease in Lactobacillus leads to reduced phosphate levels in the surrounding intestine and an increase in collagenase-producing bacteria. These bacteria release collagenases that activate inflammatory responses, further increasing the AL risk. Therefore, incorporating non-absorbable oral phosphate supplementation into bowel cleansing protocols may help mitigate these effects (142).
Microbiome restoration
Animal studies have shown that a diverse microbiome significantly promotes intestinal anastomotic healing, whereas germ-free mice or those with a single bacterial colony exhibit poor healing (62,143). Probiotics are preparations of live microorganisms that provide health benefits when consumed in sufficient quantities. They are mainly composed of species from the Bifidobacterium and Lactobacillus genera (144). These beneficial microbes enhance the intestinal epithelial barrier in both mice and humans, reducing infections in patients with colorectal cancer after colon surgery and strengthening gut mucosal barrier integrity (145). Studies on perioperative probiotic use, particularly with Bifidobacterium, have reported a reduction in harmful bacteria, including Enterobacteriaceae, Clostridium difficile and Pseudomonas, postoperatively (144). However, while animal studies suggest that probiotics and synbiotics promote anastomotic healing, clinical evidence supporting their effectiveness as standalone treatments remains inconclusive. A network meta-analysis attempted to identify the most effective intervention between prebiotics, probiotics, synbiotics or oral antibiotics for reducing infection rates in patients undergoing elective colorectal surgery. It was demonstrated that oral antibiotics were the most effective in reducing both infection rates and AL rates. While synbiotics and probiotics appeared to reduce postoperative infections, they did not significantly reduce AL rates (146).
Fecal microbiota transplantation (FMT), also known as fecal bacteriotherapy, involves transplanting stool from a healthy donor into the recipient's gastrointestinal tract to restore gut microbiota diversity and balance (147,148). Mice that received FMT from patients with AL exhibited impaired anastomotic healing. In an experimental setup, antibiotic-conditioned mice received FMT from patients with AL prior to surgery. These results showed that these mice had compromised colonic healing, characterized by lower concentrations of extracellular matrix components and higher concentrations of E-cadherin, indicating impaired cell migration at the wound edges (138). The aforementioned study collected preoperative fecal samples from 77 patients with colorectal cancer. Among them, 9 patients subsequently developed anastomotic leakage (AL), who were then matched with 9 non-AL patients by age, sex, and tumor location (non-AL vs. AL groups). Through FMT, the preoperative fecal samples were transferred to antibiotic-pretreated mice, which subsequently underwent surgical procedures.
ERAS
A key strategy for minimizing perioperative microbiome disruption is the implementation of ERAS protocols. ERAS aims to reduce surgical stress responses and organ dysfunction, leading to fewer postoperative complications and faster recovery. Emerging consensus guidelines emphasize early mobilization, immediate resumption of food intake and reduced opioid analgesia use, all of which help maintain a healthier microbiome and reduce postoperative bacterial growth (149,150). ERAS protocols recommend preoperative oral carbohydrate drinks to improve nutritional status and early reintroduction of oral intake after surgery. SCFAs such as butyrate, propionate and acetate, primarily produced through the fermentation of complex carbohydrates like fiber, may exhibit significant metabolic and protective effects. SCFAs serve as primary energy sources for intestinal epithelial cells, activate GPCRs and inhibit histone deacetylases, thereby promoting gut barrier integrity (Table II) (151).
Table IITherapeutic interventions for preventing anastomotic leakage from a microbiome perspective and their mechanisms. |
9. Conclusion
Despite advancements in surgical techniques, AL rates have not significantly decreased in recent years. AL arises from a complex and dynamic interplay of multiple factors and biological processes, including gut microbiome dysbiosis, inflammation, host genetics, immune responses and pathogenic microbes capable of compromising the intestinal barrier. Future therapeutic strategies should focus on fostering and maintaining a diverse and balanced microbiome rather than merely eradicating specific microbes. Interventions that mitigate collagen degradation, reduce free radical production and regulate control matrix metalloproteinase activation may prove more effective compared with antimicrobial treatments alone. ERAS protocols, which reduce hospital stays and healthcare costs, offer a promising framework for improving surgical outcomes by addressing microbiome-related factors. Microbiome-based biomarkers must be developed and validated to predict AL risk preoperatively, enabling targeted interventions for high-risk patients. Microbiome-modulating drugs, such as probiotics, prebiotics, synbiotics or targeted antibiotics, must be investigated and designed according to the unique needs of surgical patients to promote a healing-friendly microbial environment. Artificial intelligence and machine learning must be implemented to analyze and predict individual microbiome profiles, enabling personalized perioperative care strategies that optimize gut microbiota composition for improved wound healing. Mechanisms through which specific microbial metabolites and immune-modulating factors influence anastomotic healing must be investigated, with a focus on collagen metabolism, epithelial barrier integrity and inflammatory responses. ERAS protocols must be combined with microbiome-targeted therapies to enhance recovery, reduce complications and improve long-term surgical outcomes. By prioritizing these research areas, understanding of the gut microbiome's role in anastomotic healing can be advanced, and innovative, personalized approaches can be developed to reduce AL rates and improve patient outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YC, NW, TS, LK and JF conceived and designed the review. YC and NW drafted the manuscript. ZZ, GO, CX and XY conducted the literature search and data analysis. TS, LK, JF and GO critically revised the manuscript for important intellectual content. All authors read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Paun BC, Cassie S, MacLean AR, Dixon E and Buie WD: Postoperative complications following surgery for rectal cancer. Ann Surg. 251:807–818. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Lee DS, Ryu JA, Chung CR, Yang J, Jeon K, Suh GY, Lee WY and Park CM: Risk factors for acquisition of multidrug-resistant bacteria in patients with anastomotic leakage after colorectal cancer surgery. Int J Colorectal Dis. 30:497–504. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Makanyengo SO, Carroll GM, Goggins BJ, Smith SR, Pockney PG and Keely S: Systematic review on the influence of tissue oxygenation on gut microbiota and anastomotic healing. J Surg Res. 249:186–196. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Nalluri-Butz H, Bobel MC, Nugent J, Boatman S, Emanuelson R, Melton-Meaux G, Madoff RD, Jahansouz C, Staley C and Gaertner WB: A pilot study demonstrating the impact of surgical bowel preparation on intestinal microbiota composition following colon and rectal surgery. Sci Rep. 12(10559)2022.PubMed/NCBI View Article : Google Scholar | |
|
Gaines S, Shao C, Hyman N and Alverdy JC: Gut microbiome influences on anastomotic leak and recurrence rates following colorectal cancer surgery. Br J Surg. 105:e131–e141. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Hartman AL, Lough DM, Barupal DK, Fiehn O, Fishbein T, Zasloff M and Eisen JA: Human gut microbiome adopts an alternative state following small bowel transplantation. Proc Natl Acad Sci USA. 106:17187–17192. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Reddy BS, Gatt M, Sowdi R and MacFie J: Surgical manipulation of the large intestine increases bacterial translocation in patients undergoing elective colorectal surgery. Colorectal Dis. 8:596–600. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Boulange CL, Neves AL, Chilloux J, Nicholson JK and Dumas ME: Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 8(42)2016.PubMed/NCBI View Article : Google Scholar | |
|
Morgan XC, Segata N and Huttenhower C: Biodiversity and functional genomics in the human microbiome. Trends Genet. 29:51–58. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Weinstock GM: Genomic approaches to studying the human microbiota. Nature. 489:250–256. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Belizario JE and Napolitano M: Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front Microbiol. 6(1050)2015.PubMed/NCBI View Article : Google Scholar | |
|
Littman DR and Pamer EG: Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 10:311–323. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Guan L and Liu R: The role of diet and gut microbiota interactions in metabolic homeostasis. Adv Biol (Weinh). 7(e2300100)2023.PubMed/NCBI View Article : Google Scholar | |
|
Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al: Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 461:1282–1286. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Ducarmon QR, Zwittink RD, Hornung BVH, van Schaik W, Young VB and Kuijper EJ: Gut microbiota and colonization resistance against bacterial enteric infection. Microbiol Mol Biol Rev. 83:e00007–19. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Parada VD, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN and Hermoso MA: Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 10(277)2019.PubMed/NCBI View Article : Google Scholar | |
|
Yan H and Ajuwon KM: Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS One. 12(e179586)2017.PubMed/NCBI View Article : Google Scholar | |
|
Kolodziejczyk AA, Zheng D and Elinav E: Diet-microbiota interactions and personalized nutrition. Nat Rev Microbiol. 17:742–753. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Huipeng W, Lifeng G, Chuang G, Jiaying Z and Yuankun C: The differences in colonic mucosal microbiota between normal individual and colon cancer patients by polymerase chain reaction-denaturing gradient gel electrophoresis. J Clin Gastroenterol. 48:138–144. 2014.PubMed/NCBI View Article : Google Scholar | |
|
DeJong EN, Surette MG and Bowdish D: The gut microbiota and unhealthy aging: Disentangling cause from consequence. Cell Host Microbe. 28:180–189. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Zheng Z, Hu Y, Tang J, Xu W, Zhu W and Zhang W: The implication of gut microbiota in recovery from gastrointestinal surgery. Front Cell Infect Microbiol. 13(1110787)2023.PubMed/NCBI View Article : Google Scholar | |
|
Zmora N, Suez J and Elinav E: You are what you eat: Diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 16:35–56. 2019.PubMed/NCBI View Article : Google Scholar | |
|
He B, Hoang TK, Wang T, Ferris M, Taylor CM, Tian X, Luo M, Tran DQ, Zhou J, Tatevian N, et al: Resetting microbiota by Lactobacillus reuteri inhibits T reg deficiency-induced autoimmunity via adenosine A2A receptors. J Exp Med. 214:107–123. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN and Garrett WS: The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 341:569–573. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Kim KA, Gu W, Lee IA, Joh EH and Kim DH: High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 7(e47713)2012.PubMed/NCBI View Article : Google Scholar | |
|
Schroeder BO, Birchenough GMH, Stahlman M, Arike L, Johansson MEV, Hansson GC and Bäckhed F: Bifidobacteria or Fiber Protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe. 23:27–40.e7. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Hale VL: mSphere of influence: Drivers of host-associated microbial community structure and change. mSphere. 6:e00248–21. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Bonilla DA, Perez-Idarraga A, Odriozola-Martinez A and Kreider RB: The 4R's framework of nutritional strategies for post-exercise recovery: A review with emphasis on new generation of carbohydrates. Int J Environ Res Public Health. 18(103)2020.PubMed/NCBI View Article : Google Scholar | |
|
van Wijck K, Lenaerts K, Grootjans J, Wijnands KA, Poeze M, van Loon LJ, Dejong CH and Buurman WA: Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: Strategies for evaluation and prevention. Am J Physiol Gastrointest Liver Physiol. 303:G155–G168. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Wegierska AE, Charitos IA, Topi S, Potenza MA, Montagnani M and Santacroce L: The connection between physical exercise and gut microbiota: Implications for competitive sports athletes. Sports Med. 52:2355–2369. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Shakhsheer BA, Versten LA, Luo JN, Defazio JR, Klabbers R, Christley S, Zaborin A, Guyton KL, Krezalek M, Smith DP, et al: Morphine promotes colonization of anastomotic tissues with collagenase-producing enterococcus faecalis and causes leak. J Gastrointest Surg. 20:1744–1751. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Wang H, Lu Y, Yan Y, Tian S, Zheng D, Leng D, Wang C, Jiao J, Wang Z and Bai Y: Promising treatment for type 2 diabetes: Fecal microbiota transplantation reverses insulin resistance and impaired islets. Front Cell Infect Microbiol. 9(455)2020.PubMed/NCBI View Article : Google Scholar | |
|
Langgartner D, Vaihinger CA, Haffner-Luntzer M, Kunze JF, Weiss AJ, Foertsch S, Bergdolt S, Ignatius A and Reber SO: The role of the intestinal microbiome in chronic psychosocial stress-induced pathologies in male mice. Front Behav Neurosci. 12(252)2018.PubMed/NCBI View Article : Google Scholar | |
|
Liu Y, Fan L, Cheng Z, Yu L, Cong S, Hu Y, Zhu L, Zhang B, Cheng Y, Zhao P, et al: Fecal transplantation alleviates acute liver injury in mice through regulating Treg/Th17 cytokines balance. Sci Rep. 11(1611)2021.PubMed/NCBI View Article : Google Scholar | |
|
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al: Linking long-term dietary patterns with gut microbial enterotypes. Science. 334:105–108. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Brook I, Walker RI and MacVittie TJ: Effect of antimicrobial therapy on bowel flora and bacterial infection in irradiated mice. Int J Radiat Biol Relat Stud Phys Chem Med. 53:709–716. 1988.PubMed/NCBI View Article : Google Scholar | |
|
Olivas AD, Shogan BD, Valuckaite V, Zaborin A, Belogortseva N, Musch M, Meyer F, Trimble WL, An G, Gilbert J, et al: Intestinal tissues induce an SNP mutation in Pseudomonas aeruginosa that enhances its virulence: Possible role in anastomotic leak. PLoS One. 7(e44326)2012.PubMed/NCBI View Article : Google Scholar | |
|
Gosavi R, Chia C, Michael M, Heriot AG, Warrier SK and Kong JC: Neoadjuvant chemotherapy in locally advanced colon cancer: A systematic review and meta-analysis. Int J Colorectal Dis. 36:2063–2070. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Guenaga KF, Matos D and Wille-Jorgensen P: Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. 2011(CD1544)2011.PubMed/NCBI View Article : Google Scholar | |
|
Jalanka J, Salonen A, Salojarvi J, Ritari J, Immonen O, Marciani L, Gowland P, Hoad C, Garsed K, Lam C, et al: Effects of bowel cleansing on the intestinal microbiota. Gut. 64:1562–1568. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Meyer J, Naiken S, Christou N, Liot E, Toso C, Buchs NC and Ris F: Reducing anastomotic leak in colorectal surgery: The old dogmas and the new challenges. World J Gastroenterol. 25:5017–5025. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Shogan BD, Belogortseva N, Luong PM, Zaborin A, Lax S, Bethel C, Ward M, Muldoon JP, Singer M, An G, et al: Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med. 7(286ra68)2015.PubMed/NCBI View Article : Google Scholar | |
|
Ohigashi S, Sudo K, Kobayashi D, Takahashi T, Nomoto K and Onodera H: Significant changes in the intestinal environment after surgery in patients with colorectal cancer. J Gastrointest Surg. 17:1657–1664. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Shogan BD, Smith DP, Christley S, Gilbert JA, Zaborina O and Alverdy JC: Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome. 2(35)2014.PubMed/NCBI View Article : Google Scholar | |
|
Nakazawa T, Uchida M, Suzuki T, Yamamoto K, Yamazaki K, Maruyama T, Miyauchi H, Tsuruoka Y, Nakamura T, Shiko Y, et al: Oral antibiotics and a low-residue diet reduce the incidence of anastomotic leakage after left-sided colorectal surgery: A retrospective cohort study. Langenbecks Arch Surg. 407:2471–2480. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Scarborough JE, Mantyh CR, Sun Z and Migaly J: Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection: An analysis of colectomy-targeted ACS NSQIP. Ann Surg. 262:331–337. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Harrell L, Wang Y, Antonopoulos D, Young V, Lichtenstein L, Huang Y, Hanauer S and Chang E: Standard colonic lavage alters the natural state of mucosal-associated microbiota in the human colon. PLoS One. 7(e32545)2012.PubMed/NCBI View Article : Google Scholar | |
|
Wu YJ, Wu CT, Zhang XB, Ou WT and Huang P: Clinical study of different bowel preparations on changes of gut flora in patients undergoing colorectal resection. Zhonghua Wei Chang Wai Ke Za Zhi. 15:574–577. 2012.PubMed/NCBI(In Chinese). | |
|
Weaver L, Troester A and Jahansouz C: The impact of surgical bowel preparation on the microbiome in colon and rectal surgery. Antibiotics (Basel). 13(580)2024.PubMed/NCBI View Article : Google Scholar | |
|
Kiran RP, Murray AC, Chiuzan C, Estrada D and Forde K: Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg. 262:416–425; discussion, 423-425. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Koskenvuo L, Lunkka P, Varpe P, Hyöty M, Satokari R, Haapamäki C, Lepistö A and Sallinen V: Morbidity after mechanical bowel preparation and oral antibiotics prior to rectal resection: The MOBILE2 Randomized clinical trial. JAMA Surg. 159:606–614. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Willis MA, Toews I, Soltau SL, Kalff JC, Meerpohl JJ and Vilz TO: Preoperative combined mechanical and oral antibiotic bowel preparation for preventing complications in elective colorectal surgery. Cochrane Database Syst Rev. 2(CD14909)2023.PubMed/NCBI View Article : Google Scholar | |
|
Morowitz MJ, Babrowski T, Carlisle EM, Olivas A, Romanowski KS, Seal JB, Liu DC and Alverdy JC: The human microbiome and surgical disease. Ann Surg. 253:1094–1101. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Tarazi M, Jamel S, Mullish BH, Markar SR and Hanna GB: Impact of gastrointestinal surgery upon the gut microbiome: A systematic review. Surgery. 171:1331–1340. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Tsigalou C, Paraschaki A, Bragazzi NL, Aftzoglou K, Bezirtzoglou E, Tsakris Z, Vradelis S and Stavropoulou E: Alterations of gut microbiome following gastrointestinal surgical procedures and their potential complications. Front Cell Infect Microbiol. 13(1191126)2023.PubMed/NCBI View Article : Google Scholar | |
|
Rolhion N and Chassaing B: When pathogenic bacteria meet the intestinal microbiota. Philos Trans R Soc Lond B Biol Sci. 371(20150504)2016.PubMed/NCBI View Article : Google Scholar | |
|
Wang J, Zhang W and Wu G: Intestinal ischemic reperfusion injury: Recommended rats model and comprehensive review for protective strategies. Biomed Pharmacother. 138(111482)2021.PubMed/NCBI View Article : Google Scholar | |
|
Guyton K and Alverdy JC: The gut microbiota and gastrointestinal surgery. Nat Rev Gastroenterol Hepatol. 14:43–54. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Fang X, Vazquez-Baeza Y, Elijah E, Vargas F, Ackermann G, Humphrey G, Lau R, Weldon KC, Sanders JG, Panitchpakdi M, et al: Gastrointestinal surgery for inflammatory bowel disease persistently lowers microbiome and metabolome diversity. Inflamm Bowel Dis. 27:603–616. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Shogan BD, Carlisle EM, Alverdy JC and Umanskiy K: Do we really know why colorectal anastomoses leak? J Gastrointest Surg. 17:1698–1707. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Gao J, Gu X, Pang M, Zhang H, Lian Y, Zhou L, Feng B, Wang G, Zhang Z, Huang H, et al: Risk factors for anastomotic leak and postoperative morbidity after right hemicolectomy for colon cancer: Results from a prospective, multi-centre, snapshot study in China. Br J Surg. 111(znad316)2024.PubMed/NCBI View Article : Google Scholar | |
|
Bachmann R, Leonard D, Delzenne N, Kartheuser A and Cani PD: Novel insight into the role of microbiota in colorectal surgery. Gut. 66:738–749. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Alverdy JC: Microbiome medicine: This changes everything. J Am Coll Surg. 226:719–729. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Honda K and Littman DR: The microbiota in adaptive immune homeostasis and disease. Nature. 535:75–84. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Souza DG, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ and Teixeira MM: The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol. 173:4137–4146. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Wynn TA and Ramalingam TR: Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat Med. 18:1028–1040. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Souza DG, Fagundes CT, Amaral FA, Cisalpino D, Sousa LP, Vieira AT, Pinho V, Nicoli JR, Vieira LQ, Fierro IM and Teixeira MM: The required role of endogenously produced lipoxin A4 and annexin-1 for the production of IL-10 and inflammatory hyporesponsiveness in mice. J Immunol. 179:8533–8543. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Van Koughnett JA and Wexner SD: Surgery. NSAIDs and risk of anastomotic leaks after colorectal surgery. Nat Rev Gastroenterol Hepatol. 11:523–524. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Yauw STK, Arron M, Lomme RMLM, van den Broek P, Greupink R, Bhatt AP, Redinbo MR and van Goor H: Microbial glucuronidase inhibition reduces severity of diclofenac-induced anastomotic leak in rats. Surg Infect (Larchmt). 19:417–423. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Bakker N, Deelder JD, Richir MC, Cakir H, Doodeman HJ, Schreurs WH and Houdijk AP: Risk of anastomotic leakage with nonsteroidal anti-inflammatory drugs within an enhanced recovery program. J Gastrointest Surg. 20:776–782. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Holte K, Andersen J, Jakobsen DH and Kehlet H: Cyclo-oxygenase 2 inhibitors and the risk of anastomotic leakage after fast-track colonic surgery. Br J Surg. 96:650–654. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Walker MR, Brown SL, Riehl TE, Stenson WF and Stappenbeck TS: Growth factor regulation of prostaglandin-endoperoxide synthase 2 (Ptgs2) expression in colonic mesenchymal stem cells. J Biol Chem. 285:5026–5039. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ and Abreu MT: Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 131:862–877. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Reisinger KW, Schellekens DH, Bosmans JW, Boonen B, Hulsewé KW, Sastrowijoto P, Derikx JP, Grootjans J and Poeze M: Cyclooxygenase-2 Is essential for colorectal anastomotic healing. Ann Surg. 265:547–554. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Makar KW, Poole EM, Resler AJ, Seufert B, Curtin K, Kleinstein SE, Duggan D, Kulmacz RJ, Hsu L, Whitton J, et al: COX-1 (PTGS1) and COX-2 (PTGS2) polymorphisms, NSAID interactions, and risk of colon and rectal cancers in two independent populations. Cancer Causes Control. 24:2059–2075. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Grainger J, Daw R and Wemyss K: Systemic instruction of cell-mediated immunity by the intestinal microbiome. F1000Res. 7(F1000 Faculty Rev-1910)2018.PubMed/NCBI View Article : Google Scholar | |
|
Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C and Mathis D: Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 32:815–827. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Lee YK, Menezes JS, Umesaki Y and Mazmanian SK: Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 108 (Suppl 1):S4615–S4622. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, et al: Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 467:967–971. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Russo E, Taddei A, Ringressi MN, Ricci F and Amedei A: The interplay between the microbiome and the adaptive immune response in cancer development. Therap Adv Gastroenterol. 9:594–605. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Ellebaek MB, Baatrup G, Gjedsted J, Fristrup C and Qvist N: Cytokine response in peripheral blood indicates different pathophysiological mechanisms behind anastomotic leakage after low anterior resection: A pilot study. Tech Coloproctol. 18:1067–1074. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Abreu MT: Toll-like receptor signalling in the intestinal epithelium: How bacterial recognition shapes intestinal function. Nat Rev Immunol. 10:131–144. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Pasare C and Medzhitov R: Toll-like receptors: Linking innate and adaptive immunity. Adv Exp Med Biol. 560:11–18. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Shi YJ, Gong HF, Zhao QQ, Liu XS, Liu C and Wang H: Critical role of toll-like receptor 4 (TLR4) in dextran sulfate sodium (DSS)-Induced intestinal injury and repair. Toxicol Lett. 315:23–30. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Burgueno JF and Abreu MT: Epithelial toll-like receptors and their role in gut homeostasis and disease. Nat Rev Gastroenterol Hepatol. 17:263–278. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S and Medzhitov R: Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 118:229–241. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Cohn I Jr and Rives JD: Antibiotic protection of colon anastomoses. Ann Surg. 141:707–717. 1955.PubMed/NCBI View Article : Google Scholar | |
|
Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N and Bruewer M: Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 296:G1140–G1149. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Mack DR, Ahrne S, Hyde L, Wei S and Hollingsworth MA: Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 52:827–833. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Delzenne NM, Cani PD, Everard A, Neyrinck AM and Bindels LB: Gut microorganisms as promising targets for the management of type 2 diabetes. Diabetologia. 58:2206–2217. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Arvans DL, Vavricka SR, Ren H, Musch MW, Kang L, Rocha FG, Lucioni A, Turner JR, Alverdy J and Chang EB: Luminal bacterial flora determines physiological expression of intestinal epithelial cytoprotective heat shock proteins 25 and 72. Am J Physiol Gastrointest Liver Physiol. 288:G696–G704. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Scales BS and Huffnagle GB: The microbiome in wound repair and tissue fibrosis. J Pathol. 229:323–331. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Rolandelli RH, Buckmire MA and Bernstein KA: Intravenous butyrate and healing of colonic anastomoses in the rat. Dis Colon Rectum. 40:67–70. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Kumar A, Wu H, Collier-Hyams LS, Kwon YM, Hanson JM and Neish AS: The bacterial fermentation product butyrate influences epithelial signaling via reactive oxygen species-mediated changes in cullin-1 neddylation. J Immunol. 182:538–546. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Wentworth CC, Jones RM, Kwon YM, Nusrat A and Neish AS: Commensal-epithelial signaling mediated via formyl peptide receptors. Am J Pathol. 177:2782–2790. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Collier-Hyams LS, Sloane V, Batten BC and Neish AS: Cutting edge: Bacterial modulation of epithelial signaling via changes in neddylation of cullin-1. J Immunol. 175:4194–4198. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, et al: Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 517:205–208. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Beutler B and Rietschel ET: Innate immune sensing and its roots: The story of endotoxin. Nat Rev Immunol. 3:169–176. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Wang L, Chen Y, Wu H, Yu HH and Ma L: Slit2-Robo4 signal pathway and tight junction in intestine mediate LPS-induced inflammation in mice. Eur J Med Res. 29(349)2024.PubMed/NCBI View Article : Google Scholar | |
|
Plociennikowska A, Hromada-Judycka A, Borzecka K and Kwiatkowska K: Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 72:557–581. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4 signal transduction pathway. Cytokine. 42:145–151. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Wang JE, Dahle MK, Yndestad A, Bauer I, McDonald MC, Aukrust P, Foster SJ, Bauer M, Aasen AO and Thiemermann C: Peptidoglycan of Staphylococcus aureus causes inflammation and organ injury in the rat. Crit Care Med. 32:546–552. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Yamada T, Sartor RB, Marshall S, Specian RD and Grisham MB: Mucosal injury and inflammation in a model of chronic granulomatous colitis in rats. Gastroenterology. 104:759–771. 1993.PubMed/NCBI View Article : Google Scholar | |
|
Dong J, Wang X, Bai G and Wang D: Research progress on the mechanisms of probiotics promoting wound healing. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 41:635–640. 2024.PubMed/NCBI View Article : Google Scholar : (In Chinese). | |
|
Chen X, Yang G, Song JH, Xu H, Li D, Goldsmith J, Zeng H, Parsons-Wingerter PA, Reinecker HC and Kelly CP: Probiotic yeast inhibits VEGFR signaling and angiogenesis in intestinal inflammation. PLoS One. 8(e64227)2013.PubMed/NCBI View Article : Google Scholar | |
|
Stappenbeck TS, Hooper LV and Gordon JI: Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 99:15451–15455. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Bosmans JW, Jongen AC, Bouvy ND and Derikx JP: Colorectal anastomotic healing: why the biological processes that lead to anastomotic leakage should be revealed prior to conducting intervention studies. BMC Gastroenterol. 15(180)2015.PubMed/NCBI View Article : Google Scholar | |
|
Thompson SK, Chang EY and Jobe BA: Clinical review: Healing in gastrointestinal anastomoses, part I. Microsurgery. 26:131–136. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Ricard-Blum S: The collagen family. Cold Spring Harb Perspect Biol. 3(a4978)2011.PubMed/NCBI View Article : Google Scholar | |
|
Singh B, Fleury C, Jalalvand F and Riesbeck K: Human pathogens utilize host extracellular matrix proteins laminin and collagen for adhesion and invasion of the host. FEMS Microbiol Rev. 36:1122–1180. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Foppa C, Ng SC, Montorsi M and Spinelli A: Anastomotic leak in colorectal cancer patients: New insights and perspectives. Eur J Surg Oncol. 46:943–954. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, Possemiers S, Van Holle A, François P, de Vos WM, et al: Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 60:2775–2786. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Wiegerinck M, Hyoju SK, Mao J, Zaborin A, Adriaansens C, Salzman E, Hyman NH, Zaborina O, van Goor H and Alverdy JC: Novel de novo synthesized phosphate carrier compound ABA-PEG20k-Pi20 suppresses collagenase production in Enterococcus faecalis and prevents colonic anastomotic leak in an experimental model. Br J Surg. 105:1368–1376. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Li H, Limenitakis JP, Fuhrer T, Geuking MB, Lawson MA, Wyss M, Brugiroux S, Keller I, Macpherson JA, Rupp S, et al: The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun. 6(8292)2015.PubMed/NCBI View Article : Google Scholar | |
|
Biedermann L and Rogler G: The intestinal microbiota: Its role in health and disease. Eur J Pediatr. 174:151–167. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Hand TW: The role of the microbiota in shaping infectious immunity. Trends Immunol. 37:647–658. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Hecht G: Innate mechanisms of epithelial host defense: Spotlight on intestine. Am J Physiol. 277:C351–C358. 1999.PubMed/NCBI View Article : Google Scholar | |
|
Bosmans JW, Jongen AC, Birchenough GM, Nyström EE, Gijbels MJ, Derikx JP, Bouvy ND and Hansson GC: Functional mucous layer and healing of proximal colonic anastomoses in an experimental model. Br J Surg. 104:619–630. 2017.PubMed/NCBI View Article : Google Scholar | |
|
van Praagh JB, de Goffau MC, Bakker IS, van Goor H, Harmsen HJM, Olinga P and Havenga K: Mucus microbiome of anastomotic tissue during surgery has predictive value for colorectal anastomotic leakage. Ann Surg. 269:911–916. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Berkes J, Viswanathan VK, Savkovic SD and Hecht G: Intestinal epithelial responses to enteric pathogens: Effects on the tight junction barrier, ion transport, and inflammation. Gut. 52:439–451. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Sun J, Hobert ME, Rao AS, Neish AS and Madara JL: Bacterial activation of beta-catenin signaling in human epithelia. Am J Physiol Gastrointest Liver Physiol. 287:G220–G227. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Jakobsson HE, Rodriguez-Pineiro AM, Schutte A, Ermund A, Boysen P, Bemark M, Sommer F, Bäckhed F, Hansson GC and Johansson ME: The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 16:164–177. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Kurashima Y and Kiyono H: Mucosal ecological network of epithelium and immune cells for gut homeostasis and tissue healing. Annu Rev Immunol. 35:119–147. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Alam A, Leoni G, Quiros M, Wu H, Desai C, Nishio H, Jones RM, Nusrat A and Neish AS: The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat Microbiol. 1(15021)2016.PubMed/NCBI View Article : Google Scholar | |
|
Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, de Vos WM and Satokari R: Akkermansia muciniphila Adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol. 81:3655–3662. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Pull SL, Doherty JM, Mills JC, Gordon JI and Stappenbeck TS: Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA. 102:99–104. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Komen N, Slieker J, Willemsen P, Mannaerts G, Pattyn P, Karsten T, de Wilt H, van der Harst E, van Leeuwen W, Decaestecker C, et al: Polymerase chain reaction for Enterococcus faecalis in drain fluid: the first screening test for symptomatic colorectal anastomotic leakage. The Appeal-study: analysis of parameters predictive for evident anastomotic leakage. Int J Colorectal Dis. 29:15–21. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Shi S, Liu Y, Wang Z, Jin X, Yan W, Guo X, Lin B, Wang H, Li B, Zheng J and Wei Y: Fusobacterium nucleatum induces colon anastomosis leak by activating epithelial cells to express MMP9. Front Microbiol. 13(1031882)2022.PubMed/NCBI View Article : Google Scholar | |
|
Singh KV, Nallapareddy SR, Sillanpaa J and Murray BE: Importance of the collagen adhesin ace in pathogenesis and protection against Enterococcus faecalis experimental endocarditis. PLoS Pathog. 6(e1000716)2010.PubMed/NCBI View Article : Google Scholar | |
|
Tomita H and Ike Y: Tissue-specific adherent Enterococcus faecalis strains that show highly efficient adhesion to human bladder carcinoma T24 cells also adhere to extracellular matrix proteins. Infect Immun. 72:5877–5885. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Schardey HM, Kamps T, Rau HG, Gatermann S, Baretton G and Schildberg FW: Bacteria: A major pathogenic factor for anastomotic insufficiency. Antimicrob Agents Chemother. 38:2564–2567. 1994.PubMed/NCBI View Article : Google Scholar | |
|
Jin Y, Yang H, Qiao M and Jin S: MexT regulates the type III secretion system through MexS and PtrC in Pseudomonas aeruginosa. J Bacteriol. 193:399–410. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Zhang YJ, Li S, Gan RY, Zhou T, Xu DP and Li HB: Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 16:7493–7519. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Patterson E, Cryan JF, Fitzgerald GF, Ross RP, Dinan TG and Stanton C: Gut microbiota, the pharmabiotics they produce and host health. Proc Nutr Soc. 73:477–489. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Hyoju SK, Adriaansens C, Wienholts K, Sharma A, Keskey R, Arnold W, van Dalen D, Gottel N, Hyman N, Zaborin A, et al: Low-fat/high-fibre diet prehabilitation improves anastomotic healing via the microbiome: An experimental model. Br J Surg. 107:743–755. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Guo MY, Liu J, Balmes P, Yanta C, Motamedi A and Phang PT: Effects of diet and antibiotics on anastomotic healing: A mouse model study with varied dietary fiber and fat, and pre-operative antibiotics. Am J Surg. 235(115766)2024.PubMed/NCBI View Article : Google Scholar | |
|
Keskey R, Papazian E, Lam A, Toni T, Hyoju S, Thewissen R, Zaborin A, Zaborina O and Alverdy JC: Defining microbiome readiness for surgery: Dietary prehabilitation and stool biomarkers as predictive tools to improve outcome. Ann Surg. 276:e361–e369. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Hajjar R, Gonzalez E, Fragoso G, Oliero M, Alaoui AA, Calvé A, Vennin Rendos H, Djediai S, Cuisiniere T, Laplante P, et al: Gut microbiota influence anastomotic healing in colorectal cancer surgery through modulation of mucosal proinflammatory cytokines. Gut. 72:1143–1154. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Hajjar R, Oliero M, Cuisiniere T, Fragoso G, Calvé A, Djediai S, Annabi B, Richard CS and Santos MM: Improvement of colonic healing and surgical recovery with perioperative supplementation of inulin and galacto-oligosaccharides. Clin Nutr. 40:3842–3851. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Steyer GE, Puchinger M and Pfeifer J: Successful clinical avoidance of colorectal anastomotic leakage through local decontamination. Antibiotics (Basel). 13(79)2024.PubMed/NCBI View Article : Google Scholar | |
|
Schardey HM, Wirth U, Strauss T, Kasparek MS, Schneider D and Jauch KW: Prevention of anastomotic leak in rectal cancer surgery with local antibiotic decontamination: A prospective, randomized, double-blind, placebo-controlled single center trial. Int J Colorectal Dis. 35:847–857. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Jones C, Badger SA and Hannon R: The role of carbohydrate drinks in pre-operative nutrition for elective colorectal surgery. Ann R Coll Surg Engl. 93:504–507. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG and Gordon JI: Molecular analysis of commensal host-microbial relationships in the intestine. Science. 291:881–884. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Mizuta M, Endo I, Yamamoto S, Inokawa H, Kubo M, Udaka T, Sogabe O, Maeda H, Shirakawa K, Okazaki E, et al: Perioperative supplementation with bifidobacteria improves postoperative nutritional recovery, inflammatory response, and fecal microbiota in patients undergoing colorectal surgery: A prospective, randomized clinical trial. Biosci Microbiota Food Health. 35:77–87. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Liu Z, Qin H, Yang Z, Xia Y, Liu W, Yang J, Jiang Y, Zhang H, Yang Z, Wang Y and Zheng Q: Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery-a double-blind study. Aliment Pharmacol Ther. 33:50–63. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Xu L, Song M, Jiang Y and Li X: Comparative effectiveness of oral antibiotics, probiotics, prebiotics, and synbiotics in the prevention of postoperative infections in patients undergoing colorectal surgery: A network meta-analysis. Int Wound J. 20:567–578. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Quaranta G, Guarnaccia A, Fancello G, Agrillo C, Iannarelli F, Sanguinetti M and Masucci L: Fecal microbiota transplantation and other gut microbiota manipulation strategies. Microorganisms. 10(2424)2022.PubMed/NCBI View Article : Google Scholar | |
|
Ng SC, Kamm MA, Yeoh YK, Chan PKS, Zuo T, Tang W, Sood A, Andoh A, Ohmiya N, Zhou Y, et al: Scientific frontiers in faecal microbiota transplantation: joint document of Asia-Pacific Association of Gastroenterology (APAGE) and Asia-Pacific society for digestive endoscopy (APSDE). Gut. 69:83–91. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Gustafsson UO, Oppelstrup H, Thorell A, Nygren J and Ljungqvist O: Adherence to the ERAS protocol is associated with 5-year survival after colorectal cancer surgery: A retrospective cohort study. World J Surg. 40:1741–1747. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Zhuang CL, Ye XZ, Zhang XD, Chen BC and Yu Z: Enhanced recovery after surgery programs versus traditional care for colorectal surgery: A meta-analysis of randomized controlled trials. Dis Colon Rectum. 56:667–678. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Liu Z, Li N, Dang Q, Liu L, Wang L, Li H and Han X: Exploring the roles of intestinal flora in enhanced recovery after surgery. iScience. 26(105959)2023.PubMed/NCBI View Article : Google Scholar | |
|
Hajjar R, Oliero M, Fragoso G, Ajayi AS, Alaoui AA, Vennin Rendos H, Calvé A, Cuisiniere T, Gerkins C, Thérien S, et al: Modulating gut microbiota prevents anastomotic leak to reduce local implantation and dissemination of colorectal cancer cells after surgery. Clin Cancer Res. 30:616–628. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Martin L, Gillis C, Atkins M, Gillam M, Sheppard C, Buhler S, Hammond CB, Nelson G and Gramlich L: Implementation of an enhanced recovery after surgery program can change nutrition care practice: A multicenter experience in elective colorectal surgery. JPEN J Parenter Enteral Nutr. 43:206–219. 2019.PubMed/NCBI View Article : Google Scholar |